I’m pleased to announce my new collaboration with The QARP – Quality Assurance Research Professionals. I have been invited as a GMP inspections expert to deliver a webinar tailored for pharmaceutical professionals from Russia, Kazakhstan, Belarus, Uzbekistan, and Armenia.
The focus of the session will be the preparation and management of GMP inspections under the regulatory framework of the Eurasian Economic Union (EAEU).
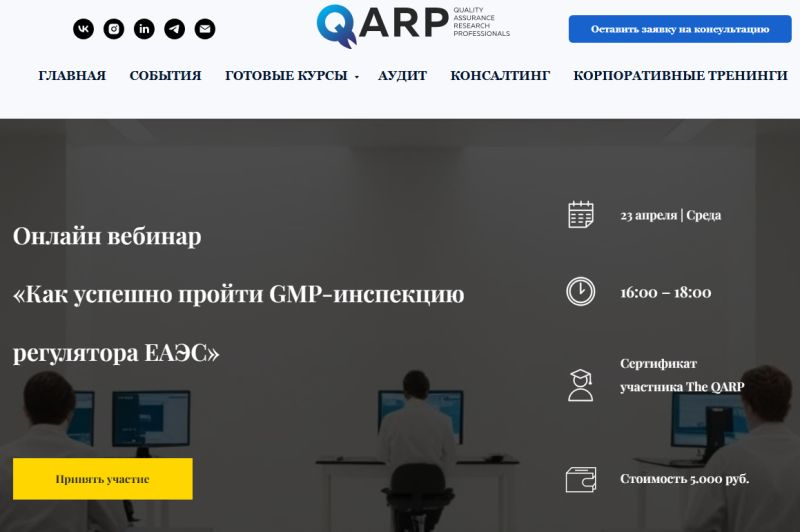
💡 Key topics we’ll cover include:
Recent regulatory updates related to GMP within the EAEU region
- Practical case studies from real inspection scenarios
- The most frequent deficiencies identified by regulatory authorities during 2024 inspections
It is an honor to be recognized as a reference in the field of GMP inspections for both human and veterinary pharmaceutical manufacturers, and to be able to share my insights with professionals in this rapidly growing and strategically important market.
- The webinar on GMP inspections will be held on April 23 at 4:00 PM (Moscow time). The language of the event is Russian.
🔍 Is your pharmaceutical company considering exporting to the EAEU and needs to prepare for GMP inspections, or do you already have registrations in these markets? I can help!
👉 I provide specialized training programs and tailored consultancy services to help companies effectively align with the latest EAEU regulatory requirements and prepare for GMP inspections.

Furthermore, through RuGMP, we provide a team of professional translators with expertise in the pharmaceutical sector to support GMP inspections and audits for manufacturers of human and veterinary medicines and medical devices, working with authorities from Russia, Kazakhstan, Belarus, Uzbekistan, Armenia, and South Korea.






